Slit lamp imaging
Purpose
General
ophthalmic observation to detect gross eye or eyelid defects (microphthalmia,
eyelid closure, bulging eye, corneal opacities, lack of pupil response to
changing light level...), followed by a detailed examination of the anterior
segment of the eye (cornea, aqueous humor, lens, and vitreous) with a thin
(slit) illumination, to better visualize anomalies in these transparent
compartments. This test does not require anesthesia, as the anterior segment
can be well-observed on vigil mice gently hand-held. Digital imaging can be
obtained from anesthetized mice.
All images taken with TEFI (Nikon DSLR + 85 mm f/1.8 lens, coupled to an Hopkins endoscopic optic).
Equipment
SL-990 Slit Lamp (CSO, Firenze, Italy)
Micron IV with slit lamp extension (Phoenix Labs, Pleasanton, CA, USA)
Reference
Hyperactivation of Alk induces neonatal lethality in knock-in AlkF1178L mice. Lopez-Delisle L(1), Pierre-Eugène C, Bloch-Gallego E, Birling MC, Duband JL, Durand E, Bourgeois T, Matrot B, Sorg T, Huerre M, Meziane H, Roux MJ, Champy MF, Gallego J, Delattre O, Janoueix-Lerosey I. Oncotarget 2014 May 15;5(9):2703-13
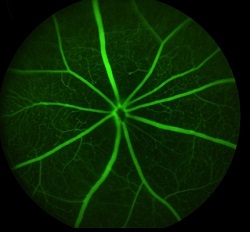
Fundus imaging
Purpose
Fundus imaging
allows detecting anomalies in retinal structure, pigmentation and/or vasculature,
as occurring in various retinal diseases as retinitis pigmentosa (RP), glaucoma
and other types of retinal degeneration. This can be combined with OCT to help precise
the localization of retinal lesions. In addition, the high sensitivity of the
Micron III allows in vivo detection
of fluorescent proteins or markers, for instance for longitudinal follow-up of
cell populations expressing GFP or RFP, or monitoring the efficacy of viral
infection (gene therapy).
Equipment
Micron IV with GFP and RFP filters (Phoenix Labs, Pleasanton, CA, USA)
TEFI
(Paques et al., IOVS 2007), which can be brought to other animal facilities if
needed due to sanitary status of the animals.
References
A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains.Simon MM, Greenaway S, White JK, Fuchs H, Gailus-Durner V, Sorg
T, Wong K, Bedu E, Cartwright EJ, Dacquin R, Djebali S, Estabel J, Graw
J, Ingham NJ, Jackson IJ, Lengeling A, Mandillo S, Marvel J, Meziane H,
Preitner F, Puk O, Roux M, Adams DJ, Atkins S, Ayadi A, Becker L, Blake
A, Brooker D, Cater H, Champy MF, Combe R, Danecek P, di Fenza A, Gates
H, Gerdin AK, Golini E, Hancock JM, Hans W, Hölter SM, Hough T, Jurdic
P, Keane TM, Morgan H, Müller W, Neff F, Nicholson G, Pasche B,
Roberson LA, Rozman J, Sanderson M, Santos L, Selloum M, Shannon C,
Southwell A, Tocchini-Valentini GP, Vancollie VE, Wells S, Westerberg
H, Wurst W, Zi M, Yalcin B, Ramirez-Solis R, Steel KP, Mallon AM, Hrab
283 de Angelis M, Herault Y, Brown SD.
Genome Biol 2013 Jul 31;14(7):R82
Mutations in lama1 disrupt retinal vascular development and inner limiting membrane formation. Edwards M.M., Mammadova-Bach E., Alpy F., Klein A., Hicks W.L.,
Roux M., Simon-Assmann P., Smith R.S., Orend G., Wu J., Peachey N.S.,
Naggert J.K., Lefebvre O., Nishina P.M. J Biol Chem 2010 Mar 5;285(10):7697-711
Retinoic acid receptor (RAR)-alpha is not critically required for mediating retinoic acid effects in the developing mouse retina. Cammas L., Trensz F., Jellali A., Ghyselinck N.B., Roux M.J., Dolle P. Invest Ophthalmol Vis Sci 2010 Jun;51(6):3281-90
Disease progression despite early loss of polyglutamine protein expression in SCA7 mouse model. Helmlinger D., Abou-Sleymane G., Yvert G., Rousseau S., Weber C., Trottier Y., Mandel J.L., Devys D. J Neurosci 2004 Feb 25;24(8):1881-7
Progressive retinal degeneration and dysfunction in R6 Huntington's disease mice. Helmlinger D, Yvert G, Picaud S, Merienne K, Sahel J, Mandel JL, Devys D. Hum Mol Genet 2002;15;11(26):3351-9
Angiography
Purpose
The
injection of a fluorescent dye (fluorescein, Evans Blue) allows a detailed
visualization of the retinal vasculature, even small capillaries, and the
detection of vascular leakage and retinal edema.
Equipment
Micron IV (Phoenix Labs, Pleasanton, CA, USA)
Optical Coherence Tomography (OCT)
Purpose
Non
invasive in vivo histology of the retina
with 2-3 µm axial resolution, allowing precise thickness measurement of all retina
layers, including photoreceptor segments. Sites (and size) of retinal lesions
can thus be identified, and their evolution tracked over time, either to
characterize a disease progression, of the efficacy of a pharmacological or
gene therapy treatment.
Equipment
Bioptigen
EnVisu R2200 (Bioptigen, Durham, NC, USA)
Electroretinogram
Purpose
Electroretinography
evaluates in vivo the activity of
retinal cells, from photoreceptors (a-wave) and inner retina neurons (b-wave,
oscillatory potentials). Activity of rod or cone circuits can be isolated by
dark- or light-adaptation of the retina, as well as changing the stimuli
frequency.
Strain background references
Equipment
Siem
Visiosystem (Siem Bio-Médicale, Nîmes, France)
References
Mutations in lama1 disrupt retinal vascular development and inner limiting membrane formation. Edwards M.M., Mammadova-Bach E., Alpy F., Klein A., Hicks W.L.,
Roux M., Simon-Assmann P., Smith R.S., Orend G., Wu J., Peachey N.S.,
Naggert J.K., Lefebvre O., Nishina P.M. J Biol Chem 2010 Mar 5;285(10):7697-711
Retinoic acid receptor (RAR)-alpha is not critically required for mediating retinoic acid effects in the developing mouse retina. Cammas L., Trensz F., Jellali A., Ghyselinck N.B., Roux M.J., Dolle P. Invest Ophthalmol Vis Sci 2010 Jun;51(6):3281-90
Disease progression despite early loss of polyglutamine protein expression in SCA7 mouse model. Helmlinger D., Abou-Sleymane G., Yvert G., Rousseau S., Weber C., Trottier Y., Mandel J.L., Devys D. J Neurosci 2004 Feb 25;24(8):1881-7
Progressive retinal degeneration and dysfunction in R6 Huntington's disease mice. Helmlinger D, Yvert G, Picaud S, Merienne K, Sahel J, Mandel JL, Devys D. Hum Mol Genet 2002;15;11(26):3351-9
Optomotor Response
The test is under improvement. We will used upgraded equipment to improve your phenotypic analysis experience ! See below the description of the older one.
Purpose
The
optomotor test is based on the reflex tracking of moving objects in the visual
field. While in humans this is achieved essentially through the optokinetic
reflex, involving only eye movements, mice tend to follow moving objects
through head movements, than can be easily visualized either in normal or low
light conditions (with IR illumination). Mice are
placed in the center of a rotating drum, which inside wall is covered with
alternating black and white stripes, with spatial frequencies ranging from 0.03
to 1.25 cycles/degree (standard spatial frequency for first line testing is
0.26 cycle/degree). Head tracking of the bar movements
is absent in blind animals. Visual acuity can be assessed by changing the bar
spatial frequency. Absence of
head tracking can have many origins. Notably albinos strains do not respond to
the optomotor test, due to a defect in projections of the ganglion cells
involved in this reflex.
Strain background references
Equipment
Reference
The optomotor response: a robust first-line visual screening method for mice. Abdeljalil Jellali, Hamid Méziane, Abdel-Mouttalib Ouagazzal, Stéphane Rousseau, Raymond Romand, Johan Auwerx, José Sahel, Pierre Chambon, Serge Picaud. Vision Res. 2005 May;45(11):1439-46.